Navigation auf uzh.ch
Navigation auf uzh.ch
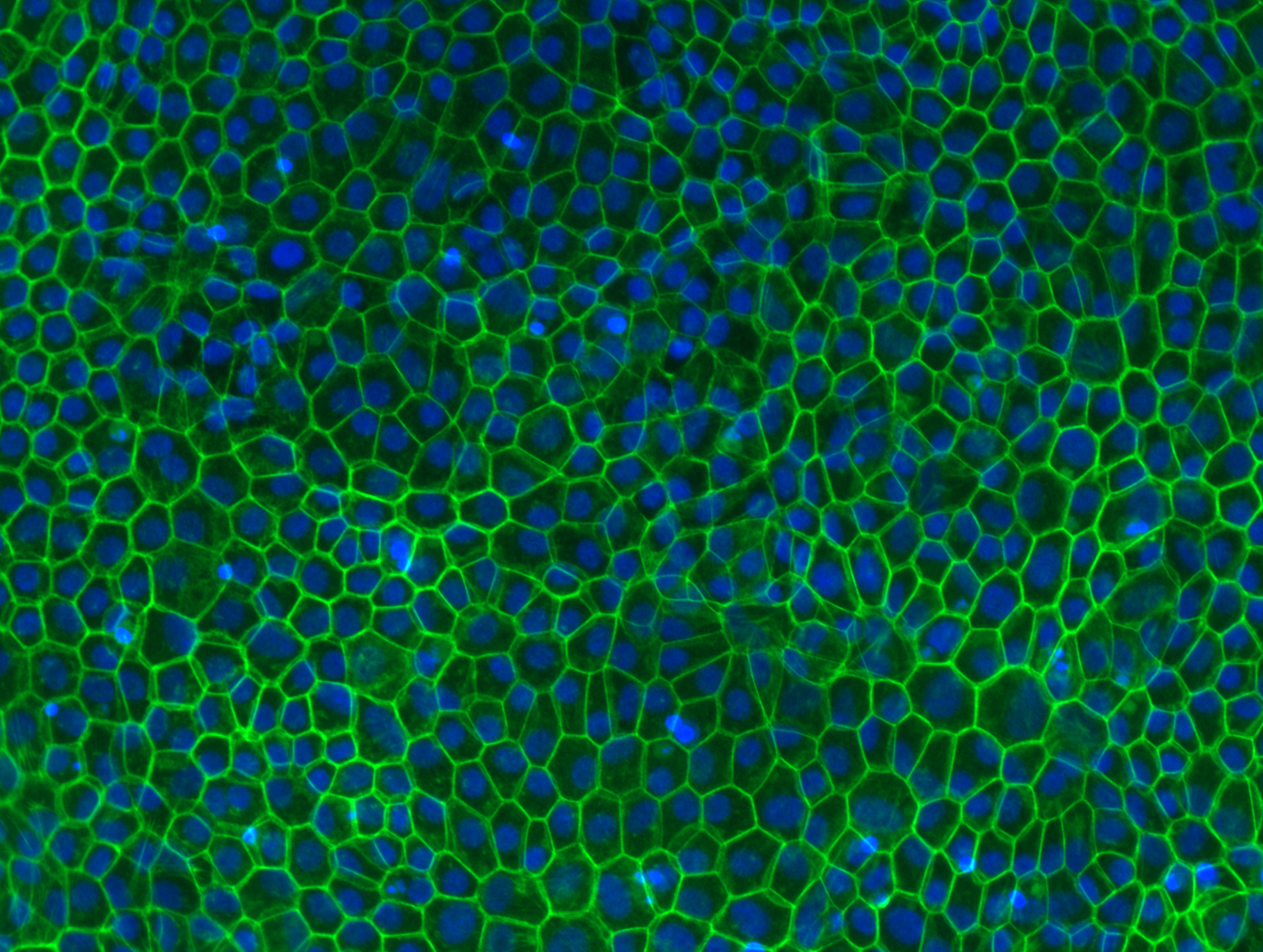
iPSC-derived retinal pigment epithelial cells stained with phalloidin (actin) and DAPI (nuclei).
Courtesy of the Grimm lab
____________________________________________________________
|
LABS |
|
|
_____________________________________________________________
|
|
Full Professor of Medical Molecular Genetics Director, Institute of Medical Molecular Genetics, University of Zurich Research Focus: Our research focuses on disease modelling of Mendelian eye disorders by making use of induced pluripotent stem cells and genome editing. Diseases include monogenic retinal dystrophies (IRDs), which are generally progressive and may lead to blindness due to dysfunction and gradual loss of photoreceptors and/or supporting retinal pigment epithelial cells. This disease family is characterized by a significant genetic heterogeneity and clinical variability and involves more than 300 associated genes and mapped loci (https://sph.uth.edu/retnet). The second group of diseases includes optic nerve hypoplasia (ONH), persistent hyperplastic primary vitreous (PHPV) and Norrie Disease (ND), the latter two of which are characterized by a maldevelopment of the neuroretina and retinal vasculature. Our disease modelling is performed using 3D retinal organoids, which are chemically differentiated from human induced pluripotent stem cells (iPSCs). In order to study the impact on vascular development, our retinal organoid model is complemented by endothelial cells differentiated from iPSC-derived mesodermal progenitors. Developmental and functional consequences of CRISPR/Cas9-mediated gene knockout or patient variant introduction in the organoid model is studied by morphologic and histologic techniques as well as gene expression approaches (mRNA sequencing (mRNA-seq) and single-cell RNA sequencing (scRNA-seq), where the latter technique provides the benefit of investigating individual subtypes of retinal cells). Other cell-based assays, including minigene assays for characterization of splicing and Luciferase reporter assays for detection of transcriptional activation, allow us to study novel genotype-phenotype associations and possibly lead to the identification of new therapeutic targets. Methods: CRISPR/Cas9 genome editing, next generation sequencing, iPSC-derived 3D retinal organoids, iPSC-derived mesodermal progenitors and endothelial cells, mRNA-seq, scRNA-seq, minigene assay, luciferase reporter assay Keywords: ATOH7, ABCA4, NDP, monogenic/Mendelian retinal diseases, vitreoretinopathies Topics: Retinal development and disease modelling Publications: https://www.medmolgen.uzh.ch/de/publications.html Website: https://www.medmolgen.uzh.ch/de.html ________________________________________________________________________top |
|
|
Professor for Experimental Ophthalmology, University of Zurich Head of Lab for Retinal Cell Biology, Dept. Ophthalmology, University Hospital Zürich Research Focus: Our research focuses on blinding diseases of the retina with a special focus on age related macular degeneration (AMD) that affects up to 25% of people above the age of 75. We use various animal models to understand specific aspects of disease etiology and progression, and develop therapeutic strategies that are based on neuroprotection and AAV-mediated gene therapy. Photoreceptor neurons form a functional unit with the underlying cells of the retinal pigment epithelium (RPE) that not only form the outer blood/retina barrier but also are crucial for photoreceptor function and survival through the regulation of metabolic support and oxygen delivery from the choroidal blood vessels to photoreceptors. Part of this regulation is the transport and recycling of lipids that originate from the daily process of photoreceptor renewal. We are using iRPE cells differentiated from iPSCs derived from patients carrying genetic variants that confer either increased or decreased risk to develop AMD. These cells closely resemble the in vivo situation in patients and are ideal to study their function in response to various stimuli and conditions that include exposure to neuronal debris with a high cholesterol load and hypoxia, an important factor for disease development. We also use CRISPR editing to inactivate genes in isogenic cells in order to study specific genetic factors for RPE function. Methods: differentiation of iPSC into iRPE cells, CRISPR/Cas9 genome editing, cholesterol efflux, phagocytosis, transepithelial resistance, single cell sequencing, transcriptomics, proteomics, AAV-based gene therapy. Keywords: RPE, lipids, hypoxia, iPSC, neurons, gene therapy Topics: neurodegeneration, gene therapy Publications: http://home.ggaweb.ch/LabForRetinalCellBiology/page1/page1.html Website: http://home.ggaweb.ch/LabForRetinalCellBiology/index.html _________________________________________________________________________ |
|
|
Principal investigator Inner Ear Stem Cell Lab Department of Otorhinolaryngology, Head and Neck Surgery, University Hospital Zurich Research Focus: Hearing is pivotal for human verbal communication, social interactions and general alertness to our surroundings. Hearing impairment as a consequence has a profound effect on the quality of life of the affected individuals. Specialized sensory cells located into the inner ear translate with remarkable speed and accuracy sound-induced vibrations of different loudness and pitch into chemical signals that can be interpreted by the brain as sound. Loss or damage of these sensory cells results in permanent hearing loss as the human inner ear cannot repair after damage. The long-term goal of our research is to develop novel therapeutic strategies to counteract sensorineural hearing loss by uncovering fundamental biological principles that underlay development and disease. We are making use of in vitro models known as “inner ear organoids” to gain insight into inner ear sensory organ development and to model disease. Further we exploit them as in vitro tools to validate novel therapeutics. Recent developments in the field of stem cell biology allow to generate in vitro inner ear sensory cell types through directed differentiation of pluripotent stem cells (PSCs). The scope of this project is to develop optimized models of human PSC-derived sensory epithelial patches and otic neurons, by leveraging recent advances in bioengineering, organoid culture and organ-on-chip technology. We aim to develop reproducible and robust in vitro models to study inner ear development, model disease and analyze drug-induced ototoxicity and otoregeneration. Methods: PSC derived inner ear organoid, cochlear progenitor culture, in vitro screening Keywords: Inner ear development, Hearing loss, Neuroscience, Disease Modeling Publications: https://www.scopus.com/authid/detail.uri?authorId=24345169900 Website: http://www.orl.usz.ch/forschung/seiten/neurootologie.aspx __________________________________________________________________________top |